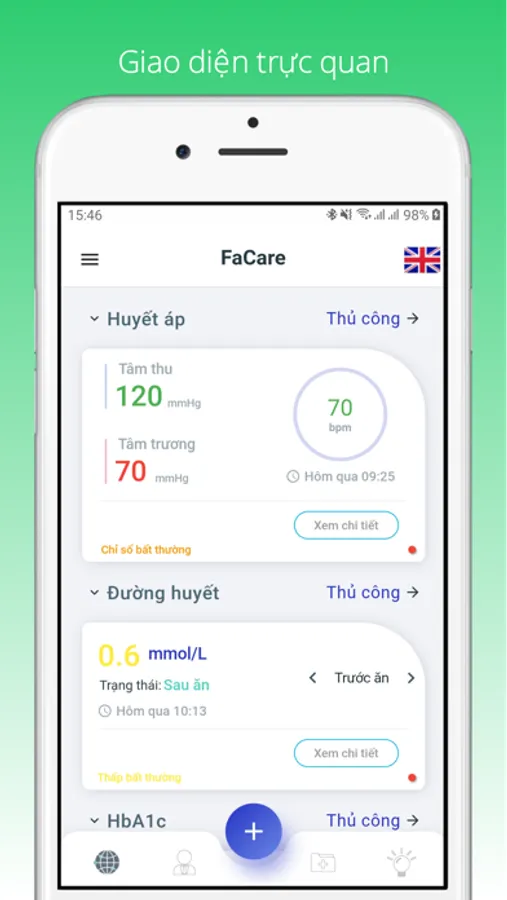
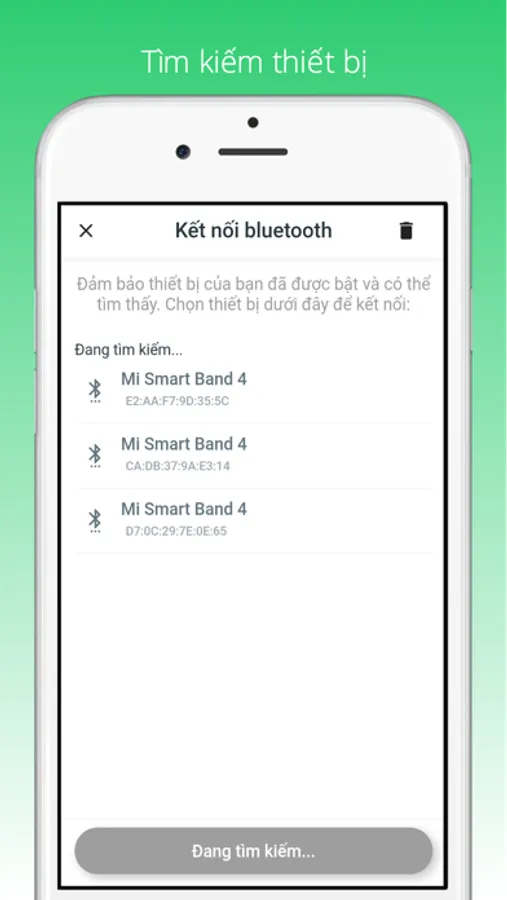
FaCare
FACARE INTERNATIONAL MEDICAL TECHNOLOGY JOINT STOCK COMPANY
Free
About FaCare
FaCare App is a personal healthcare managemnt application, that uses to storing medical examination results such as: blood pressure, heart rate, blood glucose, uric acid, total cholesterol, temperature, SPO2, hemoglobin, blood flow velocity, height and weight,... notify abnormal results to users and family doctors. Those results will be used by hospitals and doctors to diagnose and treat.
*** Notes:
- The diagnostic recommendations on the application are for reference only (we are not responsible if any problems occur). Please see your doctor or health facility before deciding any problems with our measurements.
- The medical devices used to connect and synchronize data into the application that we provide come from the brands: FaCare, TaiDoc, Fudakang, Charder. These devices all meet ISO standards and have CE/FDA certification. For details of these devices, please refer to the websites: facare.vn, taidoc.com, fudakang.com, oserio.com.
*** Regulatory Jurisdiction Statement:
This application is designed to be able to interface with medical devices manufactured by FaCare, TaiDoc, Fudakang, Charder. Each device is authorized for sale and use solely within the jurisdictions where its manufacturer has obtained the appropriate regulatory clearance or certification. Details are as follows:
- FaCare devices: Cleared for use in the European Union (CE-marked), VietNam and Taiwan.
- TaiDoc devices: Cleared for use in the European Union (CE-marked), United States (FDA-cleared), VietNam and Taiwan.
- Charder devices: Cleared for use in the European Union (CE-marked), Vietnam and Taiwan.
- Fudakang (FDK): Cleared for use in the European Union (CE-marked), United States (FDA-cleared), VietNam and Taiwan.
Users outside these jurisdictions should note that the medical hardware may not have regulatory approval in their region, and the app should not be used for diagnostic or clinical purposes in such locations.
*** Notes:
- The diagnostic recommendations on the application are for reference only (we are not responsible if any problems occur). Please see your doctor or health facility before deciding any problems with our measurements.
- The medical devices used to connect and synchronize data into the application that we provide come from the brands: FaCare, TaiDoc, Fudakang, Charder. These devices all meet ISO standards and have CE/FDA certification. For details of these devices, please refer to the websites: facare.vn, taidoc.com, fudakang.com, oserio.com.
*** Regulatory Jurisdiction Statement:
This application is designed to be able to interface with medical devices manufactured by FaCare, TaiDoc, Fudakang, Charder. Each device is authorized for sale and use solely within the jurisdictions where its manufacturer has obtained the appropriate regulatory clearance or certification. Details are as follows:
- FaCare devices: Cleared for use in the European Union (CE-marked), VietNam and Taiwan.
- TaiDoc devices: Cleared for use in the European Union (CE-marked), United States (FDA-cleared), VietNam and Taiwan.
- Charder devices: Cleared for use in the European Union (CE-marked), Vietnam and Taiwan.
- Fudakang (FDK): Cleared for use in the European Union (CE-marked), United States (FDA-cleared), VietNam and Taiwan.
Users outside these jurisdictions should note that the medical hardware may not have regulatory approval in their region, and the app should not be used for diagnostic or clinical purposes in such locations.